EDEXCEL Winter 2005 Paper 3B Question 3
This is an enthalpy reaction study where a precipitation reaction is performed and it is possible to calculate its molar enthalpy change. At first hand you might easily assume a titration study because a burette is used. But a burette can be used for measuring out accurate volumes of a liquid at any time. And such is its purpose here.
Interesting also is that the concentrations of both solutions to be mixed are given.
But read carefully first.
![]()
So by reacting known volumes of known concentrations of an alkali (… hydroxide) and sodium salt… we can produce the copper hydroxide.


Let us look further.
As soon as you see a burette you will think of titration.
And you read the instructions.
Where is endpoint?
Where is indicator?
As you will be all stressed out for the exams. You might become confused.
PANIC!!
But read carefully the instructions of the experiment.
Underline important facts.
l 20 ml copper salt soln. (1M concentration)
l Thermometer with 0.1 oC graduation è very accurate!!!
l Cup is polystyrene with lid as seen in diagram so very little heat lost.
l Now, NaOH (2M concentration) is used 2 ml at a time. Volume will be accurate! Because burette used.
And so the experiment was performed and the results plotted out!
So now lets go answer some questions.
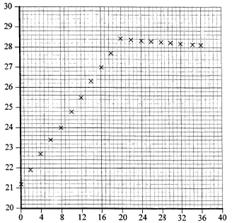
You have a graph to study. The y-axis is the temp degree C whilst the x-axis is the volume of aqueous sodium hydroxide used in cm3.
For 2 marks you are now asked to explain
Common sense really. The reaction was exothermic and when it reached the maximum the reaction is completed as there are no more copper ion left in the solution to react. But why fall slightly after further addition. No more heat energy released but whatever heat energy released has to dissipate to the rest of the cooler sodium hydroxide that is freshly added.
Now next question.
![]()
Easy really. You might want to draw line of best fit for both lines. Where they intersect there will be the volume.
You can read off the graph without drawing lines though.
Easily 20.0 cm3
Now for 1 mark,
![]()
2 Molar means 2 moles in 1000 cm3
So, in 20 cm3 you will find (2.00 x 20/1000) = 0.0400 moles of NaOH BRAVO!!!
For the next mark, …
![]()
You might think, AH! Since I know the equation since Year11 I can easily work it out from my answer in (iii) above. NO!!!!
You already know the volume and the concentration so you work out from those.
1 Molar copper salt solution mean 1 mole in 1000 cm3
So, in 20 cm3 ……sorry did you say how I know it is 20 cm3 … please look at first instruction of the experiment above.
Thank you!
Stay awake and pay attention.
So, in 20 cm3 …you will find (1.00 x 20/1000) = 0.0200 moles of Copper ions BRAVO!!! again
Phew! Whats next.
![]()
No sweat. From (iii) and (iv) ,
Cu2+ :
0.02:0.04 that means 1:2.
Easy 1 mark.
![]()
It is now possible to write the formula which will be Cu(OH)2
WOW you have experimentally discovered and proven the chemical formula of copper hydroxide. Of course empirically.
The next section involves energy calculations. Let see…

Easily you can read off the graph about 7.1 or 7.2 oC (K for Kelvin)
And you will need this value to be accurate as possible for the next question.

Easily you should get 1209 or 1210 Joules (to 3 significant figures).
Or 1.21 kJ
HORROR of horrors!! What are they now asking for? Now for 3 marks that would be tough.
So you ignore the question and go home. Whats cooking?
Come now have a go. Clue is given a(iv) and b(ii).
From a(iv) 0.0200 moles involved with release of 1.21 kJ (calculated in b(ii))
So had it been 1 mole then (1.21 x 1.00 / 0.02) = 60.5 kJ
Is the answer. But the reaction is exothermic so the sign must be negative.
Answer is thus -60.5 kJ
Oh by the way 1 mark for units, 1 mark for sign and the last from the calculations and correct answer
PHEW!!
So what is left. This is never ending.

Okay let us look at diagram and read the instructions again. What does it NOT say?
Was stirring mentioned? No!
So there you are mention that there was no stirring and the remedy to that is to stir it when added sodium hydroxide.
Now that is 2 marks!!
You can also suggest that the temp of both solutions before mixing may not have been the same so they need to be stabilized first.
BRAVO you have worked out the formula of copper hydroxide and also the molar enthalpy of the reaction. But within the allotted time?