EDEXCEL Summer 2005 Practical 3A/02 Question 1
The titration test is no doubt a must experimental activity at AS level. It is a basic method used to determine the concentration of an unknown based on knowing the concentration of a known chemical reacting with it.
Without much ado and talk about the method itself let us look at the reaction of interest.
Look below what chemicals are provided. Phosphoric acid with the formula given is in fact a triprotic acid making it very acidic. A mole of which is three times more acidic than hydrochloric acid. Because it can deliver three times more H+ ions.

But look!! The concentrations of both are given. So what is the whole point of the exercise??
Well just stick to the instructions and perform the necessary tasks.
And we shall see the aim unfurl.

Cleanliness and priming are key issues if you want to get accurate results.
To be clean the burette, pipette and other glasswares used must not contain any traces of previously used chemicals. They interfere with the reaction and hence the results.
Washing with detergent and scrubbing (difficult with burette and pipette in which case detergent plus a good deal of shaking) and rinsing with lots of tap water. Finally with distilled water as we do not wish to leave unsightly salt marks on otherwise crystal clear glass.
By priming we rinse out the burette or pipette with the solutions we want to use them for. Any traces of unwanted water or other interfering chemicals hopefully would have been reacted away and can be drained off. That is the whole purpose of the instructions given in 1 and 2 above.
You are instructed to titrate with phenolpthalein. This will require a lot of practice to decide when the end point has been reached. When it is possible to just see the tinge of pink that is when you stop and take the reading. And it has to be the same intensity of tinge of pink that we see each time. If it is strong pink then you have overshot.
You will be given a table to fill in and instructed to titrate until you get 2 values that differ no more than 0.2 ml at which point just stop as you have other tests to perform.
Below are my results.
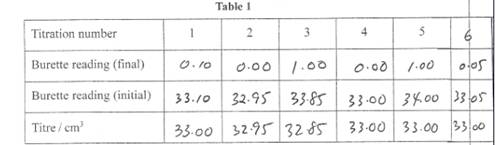
Three consecutive and exactly the same results boosts confidence in the accuracy.
Note that you do not have to start from 0.00 ml all the time. Although most of us will prefer it because it appears neat when written down. Also it helps us to slow down when we reach the end point.
Your results will be compared with the supervisors results and the further away you are then the less the marks you get.
If your marks are
well within + and – 0.2 of the supervisors results then you get the full 8
marks.
If your marks are
within + and – 0.3 of the supervisors results then you get 7 marks.
…
If your marks are
within + and – 1.00 of the supervisors results then you get only 8 marks.
Also if the student produces results and choose those far apart from each other, those that exceed 0.2ml, then further deductions will be made. The moral of this story is do it accurately!!
You are next required to list down which values you choose for your calculations.

Then calculate out the mean.
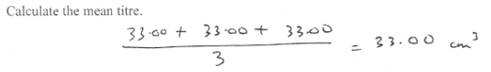
Then fill in your mean value in the right space. Note that the working out sentence has already constructed for you. You only need to fill un the values. Aren`t they nice.!!

Wow!! Look!! At the marks you will get!! Why throw it away??
![]()
Now comes the interesting part. Calculations.
At this point I will suggest the VISUALISING approach as to memorizing equations and formulas. Because of accuracy and you are less likely to make mistakes.
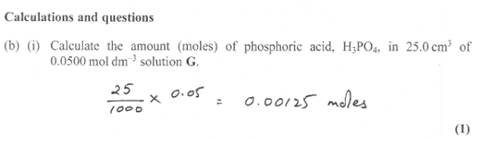
Visualise a 1 litre volumetric flask for example.
Now visualize a small 25ml volume.
Imagine and visualize that the phosphoric acid are simply particles equally distributed in the 1 litre and now you want only 25ml.
It makes sense that the no of particles in the 25ml would only be (25/1000 the number) found in 1 litre. Simply a fraction.
Yep only 1 mark WITH ALL necessary calculations to be shown!! You have to work for me.
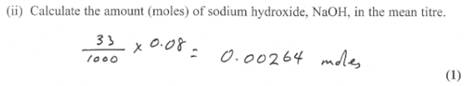
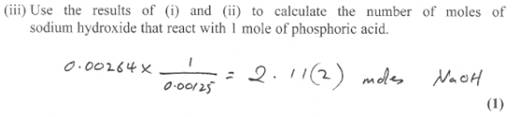
Now the fun part. Why are you asked to find out the number of moles actually reacted? When you already know their concentrations.
The reason lies in the triprotic nature of phosphoric acid. It can therefore lose only one, two or three protons in reacting and produce three different type of salts.
The ratio calculated indicated 1 mole phosphoric acid reacted with 2.11 moles NaOH. In other words 1:2.
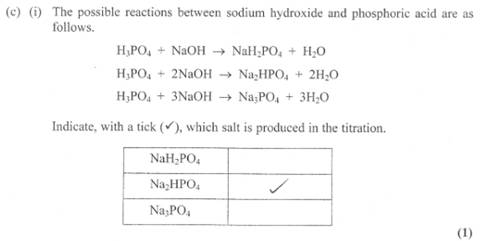
Prediction is therefore requested for just replacing one or three protons.
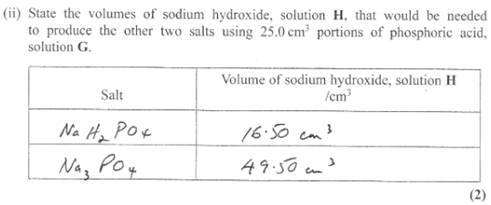
Phew! It is fairly interesting activity.
The salt formed seemed to be disodium hydrogenphosphate before phenolphthalein turned slight pink. Why?