EDEXCEL Summer 2003 Paper 6B Question 3
Organic synthesis!! I am sure you will like this.
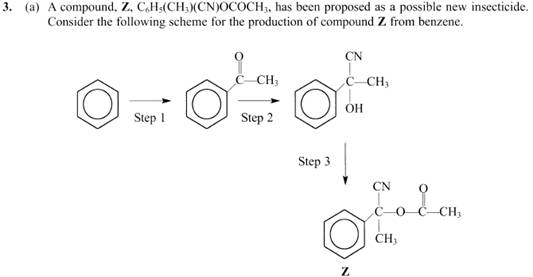
Ambitious isn`t it? You can now start your own little business synthesizing and manufacturing insecticide. Actually not so easy because you have to test if it is safe for animals and humans and if there are long reaching consequences of your use. Also if it is biodegradable.
But look at step 1. Remember the benzene ring? It is rich in electrons above and below the plane and is negatively charged. So for a bond to form a positively charged species has to move in. The best candidate is ethanoyl chloride.
The process is called ‘acylation’.
Now let us look at the question and for 3 marks.
![]()
It seems that you need to know the conditions fairly well.
Looks difficult to begin with but hang on… in step one reaction is with a benzene ring whereas… in step 3 reaction is like with an alcohol ‘R-OH’. Ethanoic acid can be used to react with this alcohol to produce an ester, the product of step 3.
Hang on… SAME REAGENT.. that’s it , why not use ethanoyl chloride in step 3, much easier for reaction to take place.
Now the tricky part, different conditions. Well a benzene (arene) ring structure is very stable due to delocalization of the electrons and adopting a resonance structure, so a lot of energy(heat) need to be given to make it easier to break a bond. Step 1 will require heating over extended time.
What about step 3? Well the reaction is not at the benzene ring itself. It is as if the reaction is with a non aromatic structure.
So heating may not be required. (It does not though)
That’s it WELL DONE!! For the same reason too a catalyst like AlCl3 required to help out with the step 1 and not required for step 3.
Next, for 5 marks now you are required to …
![]()
Looking at it carefully we have introduced a cyanide group!
This should be easy but now you have to give the reagents, explain by drawing and naming the mechanism.
That is really 3 requirements.
Reagents: Potassium Cyanide and Hydrochloric acid then add sodium hydroxide (buffered) to pH 6-8. This will ensure plentiful CN-
Mechanism: Nucleophilic Addition
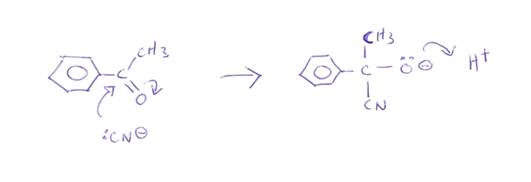
Note that Oxygen is more electronegative than carbon so draws electrons to itself and away from the carbon atom making the carbon nucleus more exposed and positively charged. The cyanide ion is negatively charged and so attacks and add on to the carbon nucleus. So it is called nucleophilic addition.
Next for 5 marks…
![]()
Well what is the strategy then.
Find out how many moles of Z in 3.00g of Z
Also how many moles of benzene in 10.0g of benzene.
Logically if ALL the benzene ring structure made through the synthetic pathway to Z then the no of moles calculated above would be the same.
So here goes. RMM of Z which is 11 of C + 11 of H +1 of N + 2 of O = (11 x 12) + (11 x 1) + 14 + (2 x 16) = 189
3.00 g of Z is equivalent to 0.0158(73) moles
And, RMM of benzene is 6 of C + 6 of H = (12 x 6) + 6 = 78
10.00 g of benzene is equivalent to 10/78 = 0.128(205)
Since the mole ratio of the multistep process is 1 : 1 then we would expect 0.128(205) moles of Z produced but we don’t!!
Yield thus = (0.0158(73) / 0.128(205)) x 100
= 12.38 %
= 12.4 % (answer to 3 significant figures)
Another approach is to find out the actual grams of Z to be produced from 10 g of benzene then work out the yield from there.
Up to you.
Let us look at the next section.
![]()
First the characters come in. Urea and ammonium sulphate..
Then a more information.
![]()
For 3 marks, why is urea soluble?
Well remember that water is a dipole. The two hydrogen atoms are slightly positive while the oxygen is slightly negative.
So for something to be soluble in water it must be either a dipole or fully ionic.
In the case of the Urea molecule the nitrogen has a lone pair of electron which thus makes it slightly negatively charged. This can easily form hydrogen bonds with the slightly positively charged hydrogen of water. Each Urea molecule also has two –NH2 functional groups. So that makes it easily soluble because hydrogen bonding with water molecules is possible at both ends of the molecule.
Now let us look at the last question.
![]()
Well, take a look. The % of nitrogen is 100 X 28/(36 + 32 + 64) = 21.21%
Compare that with ammonium nitrate 100 X 28/(18 + 14 + 48) = 35%
So for every 1 kilogram that you buy and use ammonium sulphate a lot of it would be wasted.
This is because of the SO42- part. When the ammonium is used up because of the nitrogen what is left would be the sulphate part.
As ammonium is highly soluble then it can leached and washed out of the soil into rivers.
Ethanoyl chloride is a very useful reagent and the acylation technique is useful in attaching a chain to an aromatic group. Ethanoyl chloride is more effective in reactions where normally ethanoic acid would have been used. In this case esterification.