EDEXCEL Summer 2003 Practical 6A/01 Question 2
This question looks pretty straightforward does it not?
Well yes. Still you have to perform the tests and hope to get some positive results. A negative result does not mean you are in the wrong just eliminating possibilities.

If you observe an orange ppt then it is very likely that the carbonyl functional group exist. Whether aldehyde or ketone. Lets say you do.

This can be puzzling. Well, because we are used to the idea that silver nitrate is used to test for chlorides at IGCSE level and also at AS level for halides in general but found in Inorganic Chemistry.
What then in Organic Chemistry? Probably a test for Halogenoalkane. But to perform the test you need to warm the sample first with aqueous sodium hydroxide to shake lose the halide then acidify with dilute nitric acid followed by adding aqueous silver nitrate. The method above is strikingly different as the reagent seemed to be freshly changed somewhat with ammonia been used.
It is in fact preparation of ammoniacal silver nitrate reagent which is then added with sample C that we know for sure has a carbonyl group BUT we do not know if it is an aldehyde or a ketone.
Lets say you have a silver mirror. So you inferred aldehyde confirmed.
You are now given a mass spec
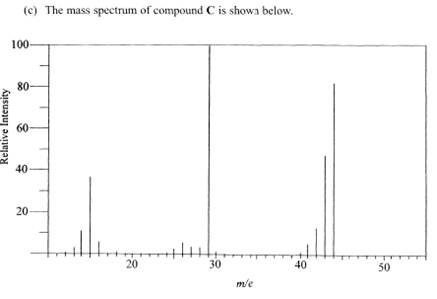
And asked to work out the ion
There is a peak at 15, 29 and 44
15 would probably be methyl- CH3-
29 possibly ethyl- C2H5- but also carbonyl with hydrogen next to it –CHO
{work our their relative molecular mass and you can see it}
44 cannot be propyl- because propyl will give m/e value of 43
It is very likely to be the original molecule intact but with an electron bumped off.
That means that the two lower m/e peaks must add up to give the higher m/e peak which it does!!!
Combining the methyl to the aldehyde group we fit CH3- to -CHO and we get CH3CHO which is ethanal.
The m/e charge would then be 44 and the structural formula CH3CHO (+)
Please REMEMBER the positive charge inside a circle !!!
You now proceed to the next chemical.

Why sodium carbonate? Remember this is Organic Chemistry not Inorganic where you will test for cations.
The only test is for carboxylic acids. In general acids react with carbonates to give CO2 and effervescence is noted. Lets say you do. So we have a carboxylic acid. –COOH or carboxylic acid functional group inferred.
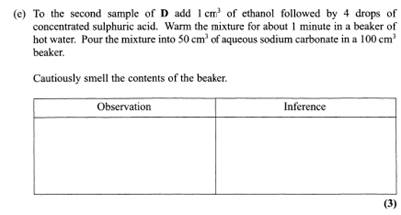
Usually when ethanol and a carboxylic acid is added and conc H2SO4 added a sweet smell of ester is detected. But why the sodium carbonate added? Obvious reason!!
Would you want to breathe in acid vapours!!??!!
NO!!
By reacting away the acid conc H2SO4 you can now safely smell the ester if produced.
Lets say you do. Interesting to note 3 instead of 2 marks as in previous questions.
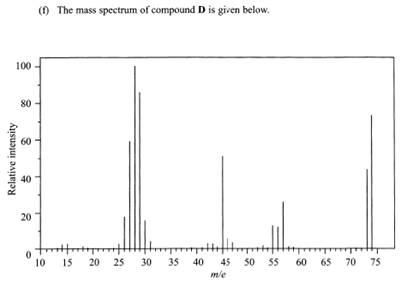
We now have another mass spec.
Look at the peaks
28, 45, 57 and 74
Looks like the molecule took a lot of thrashing.
A long chain without side branch would break easily. Imagine whacking a string of beads.
As we detected carboxylic acid 74 would possibly be CH3CH2COOH(+)
45 could be –COOH(+)
There is 29 which could be the CH3CH2- bit that complements –COOH(+)
But there is more of 28 than 29. Is that a problem?
Maybe CH3CH2-(+) has lost a H(+) ?? The mass spec does not include H(+) in it so no comments. Still there is a medium sized peak at 73 ?!?
57 could be CH3CH2CO(+) with OH(+) fallen off but where is peak at 17 ?
Time is running out perhaps better to settle for propanoic acid so m/e is 74 and CH3CH2COOH(+) is the structure.
SO WHAT IS THE ANSWER?
C is ETHANAL and D is PROPANOIC ACID