Discussion of Paper
6, Nov2006, Q7on Spectroscopy
Chemistry CIE
9701,
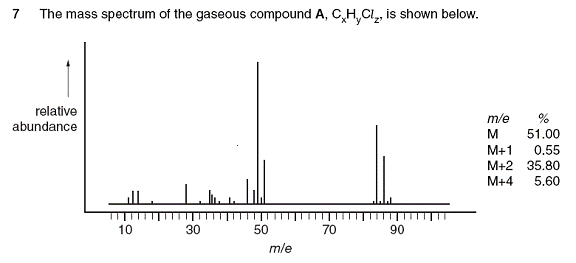
This is a mass spectroscopy question and should be easy.
The questions to answer are :-
It is difficult to tell which question will gain 2 marks. If in doubt then make sure that your answers are sufficiently accurate and detailed.
(i) As the graph does not specifically say what the peaks M, M+1 etc. are, one would assume that the peaks on the far right would be the ones. The m/e and %s does offer an idea to the shape of peaks. The group on the right seems to fit the data.
So what causes the peaks at 84, 86 and 88?
Well ISOTOPES of course. We are told that the chemical is CxHyClz that means 3 types of elements. C, H and Cl.
We know that chlorine has Cl35 and Cl37 isotopes. Carbon has C12, C13 and C14. Hydrogen has H1, D2 and T3.
So the combination possibilities are quite a lot.
(ii) The ratio of peaks at 49 and 51 is just about 3:1. This looks like the ratio between Cl35 and Cl37 isotopes found naturally. But why do the peak pattern between the relative peaks in the 46 to 51 range different from that between 82 to 88 ?
(iii) What then is the identity of A?
Well we can look at the highest peak in the mass spectrograph. Since it must have at least 1 C and that means up to 3 hydrogen atoms to go with the carbon what we can try is to fit the number of chlorine atoms. We can have up to 2 Chlorine atoms (Isotope Cl35) giving us approximately 70. So add 1 carbon and 2 Hydrogen atom will give us 84. Spot on.
The identity is probably CH2Cl2
AHA !!
The bells are ringing!!
There are 2 Chlorine atoms. So you can actually have CH2Cl352 , CH2 Cl35 Cl37, CH2 Cl372
Their RMM would be 84, 86 and 88.
To be more accurtate we can use the mathematical formulae below:
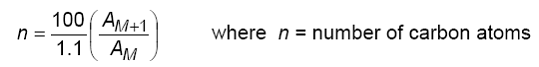
AM+1 will be 0.55 and the AM will be 51.
We can see that from the peak pattern details on the graph.
So answer will be 1.
Only one carbon atom.
Guess you just have to memorise
this formula.
![]()
Small peaks can only mean that there are isotopes that exist in small percentages. Can only be caused by C-13 or Deuterium-2. The clue however is that value of 0.55%. It cannot then be D-2 because there is very little D-2 around. It has to be C-13.
![]()
Quite a headache this question. You either know or don’t. or can just spot it.
Try all possible RMM. For methane which is 16, for ethane which is 30 and so forth. Not RMM of value 28. So what can it possibly be. Well our planet is 78% ___? Yes in fact nitrogen with RMM = 28. Easy for Nitrogen to get in and contaminate. Still unless we use this machine all the time it is difficult to spot the answer.
![]()
You really need to know the relative abundances of Br79 and Br81. Do you? Do I???
This you have to know from studying or experience in doing questions.
Well it is in fact 1:1
Easy huh?
So the possible structures and their RMM would be CH2Br792, CH2 Br79 Br81, CH2 Br81 Br79 and CH2 Br812. Since there are equal abundances of Br79 and Br81 then their relative abundances must be 1:1:1:1.
Their RMM would be 172, 174, 174 and 176.
But hang on!!
CH2 Br79 Br81, CH2 Br81 Br79 are really the same and have the same RMM of 174.
So … So…the relative peak abundances will appear as three peaks M: M+2 : M+4 and their ratio would be 1:2:1
Comparing the M+4 it would also be
distinctively higher.
It is already higher than the other small peaks in the case of CH2Cl2
so one would expect CH2Br2.
And that
brings us to the end of this question.
HOME | Chemistry A Level Home| EBooks| Contact| About