Discussion of
Paper 6, Nov2006, Q5 on Gas Liquid Chromatography, Chemistry CIE 9701
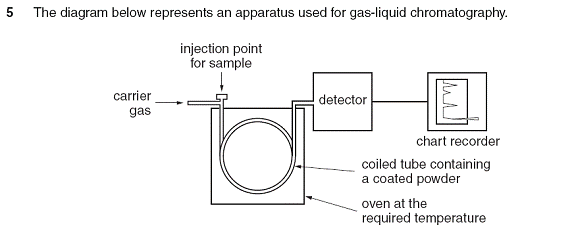
This question is indeed a straightforward question on an analytical method. Known as GLC for short.
It can take up to 1 hour to prime it, get it going at the optimum or required stable conditions. I remember my biochemistry research days. That was a long time ago though.
So what are the questions.

Now for 5 marks you have to answer (i) and (ii).
(ii) is definitely easy.
It can “only be” and “must be” an inert gas.
The principle is that we do not want any reaction to take place between the carrier gas and the chemicals we wish to separate.
Nitrogen is the best answer.
Helium and argon also possible but you have to bear the cost.
So 1 mark.
That means the other 4 marks must come from part (i).
How does it work?
Very much like Paper chromatography (if you find it hard to remember details of GLC) remember the same principles.
You definitely need :-
(a) a stationary phase (something that stays put)
(b) a mobile phase (something that moves)
So mention them for 2 marks
Don`t forget the examiner will be looking for new words you may have learnt.
Instead of just saying that components in a mixture are separated as they get pushed along (or move along) the tube with the stationary phase, you can say “the mixture of different chemical components are PARTITIONED between the stationary phase which is packed inside the column and the carrier gas which forms the mobile phase..
NOTE: The column is packed with
finely ground diatomaceous earth, which is a very porous rock.
This is coated with a high boiling liquid - typically a waxy polymer.
Now to mention the partitioning is 1 mark!!!
So far 3 marks.
For further marks then explain how the partitioning works. Every
chemical has different adsorption properties. Different chemical component will
be
held more or less strongly on the stationary phase than others. To mention that you can get 1 mark.
Also it is
important to mention the stability of the oven temperature. Otherwise there
will not be a consistent and repeatable separation. That can also offer 1 mark.
For this part
though you can only get a maximum of 4 marks. Thus 4 important points mentioned
WELL will help you get your marks.
You have to think
what common crimes exist.
Murder? Drink driving? Of
course they have other methods for the latter.
Specific chemicals
found in the crime scene?
What about the
anti-narcotics department?
Rather straightforward
question if you read up on this subject.
Drugs in Blood and
urine can be detected.
Alcohol
also.
But perhaps you
may or may not have missed out on explosives.
They are still
chemicals though and they can be detected.
Any 2 for 2
marks!!
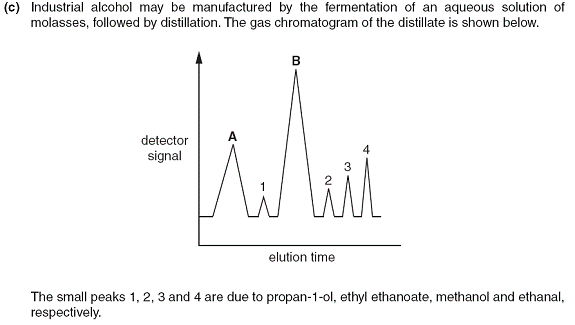
(i) Well is there a trick here?
Lets look at what reaction there is. Fermentation! So there must be sugar to begin with. And since fermentation has taken place and usually we get alcohol we would expect ethanol. In other words the products.
Looking at the list of already identified peaks we find ethanol absent. So one of the peaks will probably be ethanol. A huge peak because it is our main product.
Although CO2 is also produced it would not be detected because it has been released to the atmosphere.
It is very tempting to look at the other chemical components. Since ethyl ethanoate is detected we might think that there is ethanoic acid. Problem is both peaks A and B are HUGE!!
So what can it be?
Since the whole fermentation process took place in a very wet environment then one of the HUGE peaks can come only from water.
Since water and ethanol have Hydrogen bonding then it makes sense to fall next to propan-1-ol on either side.
PHEW!! That’s tricky.
And just for 1 mark!
(ii) is easy. The lighter and smaller molecules and also less slowed down by the column usually come out first. So the rightmost components come out first.
We can also say
eluted is first on the right. This is 1 mark.
Reason is due to
strength of bonding of chemical component to stationary phase.
It is also
acceptable to say that they have different boiling points so the one with lower
boiling point gets eluted first. However the better answer to make reference to
bonding to the stationary material. That is 1 mark!!
That’s another question
done!!
Home| Chemistry A Level Home | EBooks| Contact| About